 Click HERE if you want to receive future “European Regulatory Updates” by email. Just provide your Name, Company, Phone Number, and the email address where you would like to receive updates.
Click HERE if you want to receive future “European Regulatory Updates” by email. Just provide your Name, Company, Phone Number, and the email address where you would like to receive updates.
Annex I of the European Medical Device Directive (http://bit.ly/M5MDD) is titled “Essential Requirements.” Most companies demonstrate that their device meets the 13 Essential Requirements (ERs) by creating an Essential Requirements Checklist (ERC). I have no idea what the origin of the ERC is, but you know that regulators love tables and checklists. This particular checklist is so commonly used that the Global Harmonization Task Force (GHTF) included an example of an ERC, called an “Essential Principles Checklist” (EPC) at the end of a guidance document on how to create Summary Technical Documentation (STED) for In Vitro Diagnostic devices (http://bit.ly/STEDIVD)—which is now maintained on the IMDRF.org website.
On September 26, 2012, the European Commission released a proposal for new EU Medical Device Regulations (http://bit.ly/EUProposal). This proposal still includes ERs in Annex I, but there are 19 ERs in the proposal. One regulatory professional recently sent me a follow-up question in response to an audio seminar I conducted in November (). Her question was, “What are the six new ERs?”
A few of the early reviews of the proposal indicated that there were no significant changes, but I have learned the hard way that you should always go to the source and verify the information for yourself (i.e. – Genchi Genbutsu). Here’s what I found:
General Requirements (ER 1-6a)
- No real change to this requirement.
- This requirement was reworded to clarify the intent (see Annex ZA of EN 14971:2012 for more info @ http://bit.ly/ISO14971-2012changes).
- It appears as though the Commission thought the current ER 3 was redundant and the requirement was addressed by ER 1 and ER 5 already.
- This is now the new ER 3, and the requirement now clarifies how Notified Bodies shall apply this requirement in cases where a lifetime of the device is not stated.
- This is now the new ER 4, and there is no real change.
- This is now the new ER 5, and the wording has been clarified.
ER6a is conspicuously missing from the proposed ERs, but don’t get excited. Clinical Evaluations are still required as part of the Technical Documentation in Annex II, Section 6.1c: “the report on the clinical evaluation in accordance with Article 49(5) and Part A of Annex XIII.”
Chemical, Physical & Biological Properties (ER 7)
ER 7.1 has one new requirement: “d) the choice of materials used, reflecting, where appropriate, matters such as hardness, wear and fatigue strength.” ER 7.2 and 7.3 remain unchanged. ER 7.4 has been simplified to what is proposed as the new, shorter ER 9. ER 7.5 is now the new ER 7.4, and the changes reflect the current status of phthalate regulations and similar issues. ER 7.6 is now the new ER 7.5, but there is no change to the content. The new ER 7.6 requires that manufacturers address the risks associated with the size and properties of particles—especially nanomaterials. The changes associated with this section will impact certain device types more than others—such as orthopedic implants.
Infection & Microbial Contamination (ER 8)
ER 8 is still ER 8, but ER 8.1 is now prescriptive regarding design solutions and the current ER 8.2 is now the new ER 10. The new ER 10 is expanded and references the new EU Regulations regarding devices manufactured utilizing tissues or cells of animal origin: Commission Regulation (EU) No 722/2012 of 8 August 2012 (http://bit.ly/AnimalTissueReg). The new ER 8.2 is a new requirement that was an oversight of the MDD, and the new ER 8.7 now clarifies that the labeling must differentiate sterile and non-sterile versions of the product; packaging is no longer an acceptable mechanism for differentiation. The balance of ER 8 remains unchanged.
Construction & Environmental Properties (ER 9)
This ER is now identified as the new ER 11, and this section is expanded. This reflects the emphasis on the need to evaluate the safety of devices with accessories, compatibility with other devices, and the affects of the use environment.
Devices with a Measuring Function (ER 10)
This ER is now identified as the new ER 12, but ER 10.2 from the current Directive appears to be missing. What’s up?
Take a look at the new ER 11. ER 10.2 is now the new ER 11.6.
Protection Against Radiation (ER 11)
This ER is now identified as the new ER 13, but there is nothing new.
Requirements for Devices Connected to or Equipped with an Energy Source (ER 12)
ER 12.1 and 12.1a are now ER 14. This section is specific to software requirements and has more detail than the current Directive. IEC 62304:2006, “Medical device software – Software life cycle processes,” is the Standard that will be expected by Notified Bodies as a reference for ER 14. ER 12.2 through ER 12.6 are now ER 15, but there is nothing new. This Section ER 12.7 and its sub-parts are now addressed by ER 16. ER 12.8 and its sub-parts are now addressed by ER 17.
Information Supplied by the Manufacturer (ER 13)
This is now identified as ER 19: “Label and Instructions for Use.” This section is simplified from ER 13 (i.e. – there are fewer sections), but this ER does not seem to be any shorter. ER 19.1 has sub-parts a-g, and this ER section incorporates the concepts previously addressed by ER 13.1, 13.2, 13.4 and 13.5. ER 19.2 is a new and improved version of the previous ER 13.3 specific to labeling requirements. This labeling section is expanded from sub-parts “a” through “n” to “a” through “q”. The UDI requirement is sub-part “h”. ER 13.6 is now ER 19.3 specific to the instructions for use (IFU). This section is expanded from sub-parts “a” through “q” to “a” through “t”.
The number of sub-parts to ER 19.3 doesn’t reflect the additional requirements for IFUs that are proposed by the Commission. The sub-sections of this part warrant special attention. Items that frequently are found missing from IFUs on the market today include:
- ER 19.3c – performance intended by the manufacturer
- ER 19.3h – installation and calibration instructions
- ER 19.3k – how to determine if a re-usable device should be repaired/replaced
- ER 19.3m – restrictions on combinations with other devices
- ER 19.3o – detailed warning information
- ER 19.3p – information about safe disposal of the device
- ER 19.3t – notice to user/patient to report adverse events
ER 18 – Use by Lay Persons
This is a short section, but the requirement is new. There are now additional requirements for products intended for use by a lay person. The Risk Management Report, Design Validation, and Clinical Evaluation Report will need to include specific evidence to demonstrate conformity with this ER. The Post-Market Surveillance Plan for these products should carefully verify the accuracy of risk estimates. Post-Market Clinical Follow-up (PMCF) Studies would be challenging in the past, but the prevalence of social media and product registration databases may facilitate conducting PMCF Studies for these products in the future.
Australia & Canada
There is also an EPC that is required by the Therapeutic Goods Administration (TGA) in Australia (http://bit.ly/EPCTGA) and by the Therapeutics Product Directorate (TPD) in Canada (http://bit.ly/CanadianSTED). If you would like to learn more about the Essential Principles of Safety and Performance you should also review the GHTF guidance document on this topic (http://bit.ly/EPSafetyPerf) on the IMDRF.org website. This 2012 version of the document supersedes GHTF/SG1/N041:2005.
I have observed approval of products where the European ERC was submitted in lieu of an EPC for Australia and Canada. I guess they are a little more rationale than some other regulators, but if you have experienced any “push back” regarding this approach please share this by posting a comment or emailing me: rob@13485cert.com.
43.133884
-72.725746

 The new grading process defined by the guidance document has a two-step process. The first step uses a grading matrix to quantitatively determine a grade for the finding based upon the impact upon the QMS and the frequency of occurrence.
The new grading process defined by the guidance document has a two-step process. The first step uses a grading matrix to quantitatively determine a grade for the finding based upon the impact upon the QMS and the frequency of occurrence.
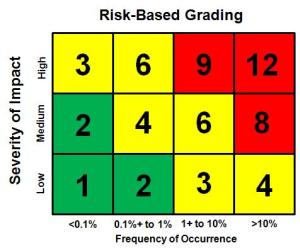 I have had enough trouble in the past with training auditors to consistently grade findings during audits, and this is one of the most important sections of the exam for a Lead Auditing Course. Recently I suggested that a client consider using the risk analysis matrix that they were already using for process risk analysis and apply the matrix to grading of findings. An example of this type of matrix is shown below.
I have had enough trouble in the past with training auditors to consistently grade findings during audits, and this is one of the most important sections of the exam for a Lead Auditing Course. Recently I suggested that a client consider using the risk analysis matrix that they were already using for process risk analysis and apply the matrix to grading of findings. An example of this type of matrix is shown below.



